KXL® System
The cross-linking solution for your keratoconus & Lasik Xtra® patients
The CE-marked KXL® system has a sleek, mobile design with a large touchscreen and wireless remote control that makes it easier to use. Together with 4 preset (changeable) protocols, a fifth user programmable treatment protocol, and the Glaukos family of high-quality riboflavin formulations, you can choose from proven and predictable protocols for your patients via epithelium-off (epi-off), accelerated, pulsed, or continuous treatments.
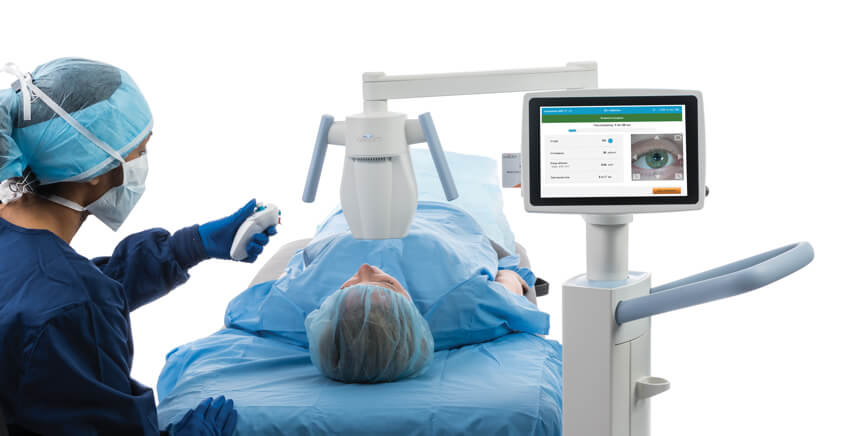
The KXL® system features:
- Up to 45 mW/cm2 of power for effective cross-linking
- Wireless remote control for accurate and convenient X, Y, and Z axis adjustments during treatment
- Large 25.7 cm touchscreen monitor for premium operator visibility and ease of use
- 4 preset treatment protocols for safety, efficiency, and accuracy
- Fifth user programmable treatment protocol for convenience
- Intuitive software user interface
- Live camera for ease of alignment and image acquisition
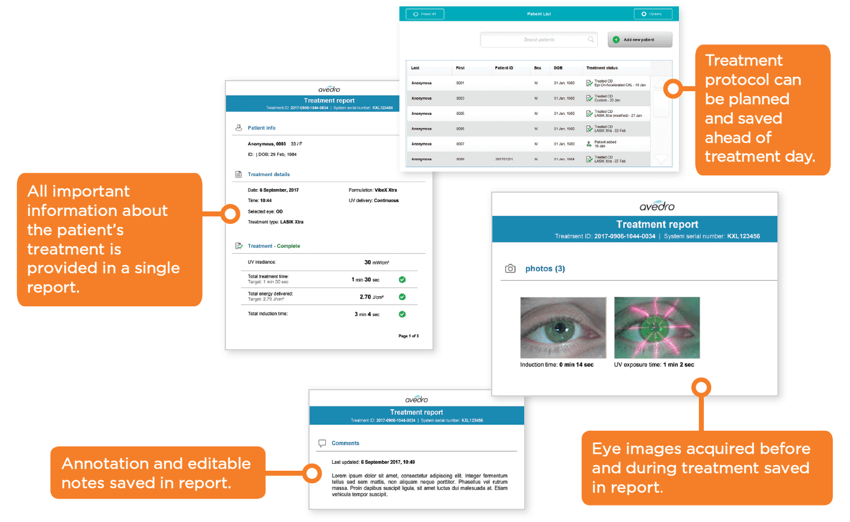
- On-board patient database for easy reference and planning
- Exportable patient treatment report for record keeping
Procedures performed with the KXL® system include cross-linking and Lasik Xtra®.
We want to hear from you!
Please contact us to learn more about our cross-linking products and procedures.
Contact Us
"*" indicates required fields
